
High Quality Huawei Mate 20 Pro Tempered Glass Curved Edge Liquid UV Screen Protectors,Huawei Mate 20 Pro Tempered Glass Curved Edge Liquid UV Screen Protectors Suppliers

Amazon.com: Huawei Mate 20 Pro (GSM Only, No CDMA) Unlocked 6GB RAM 128GB Storage Dual Sim LYA-L29 - International Version/No Warranty - Black : Cell Phones & Accessories

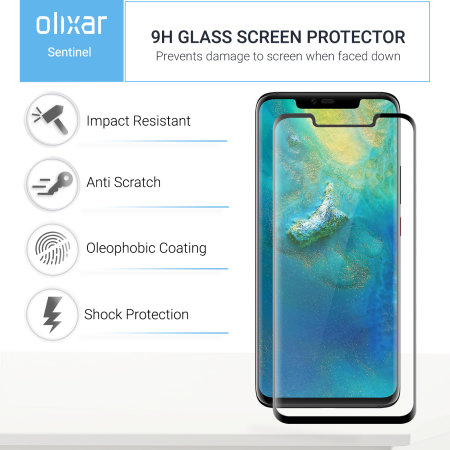











/cdn.vox-cdn.com/uploads/chorus_asset/file/13324663/vsavov_mate20pro22.jpg)





